Tuesday, February 16, 2016
After Test
After the test I'm glad I did the practice test. It was also very helpful having the ACT program on my calculator to help with the ICE box questions. I wish there was more time to go over questions, so I am going to work on my time management for our next test.
Extra Links for Studying
Our test is coming up and Frankenberg said it will be one of the hardest test we take. Due to this statement I have posted some extra links to help. I have also done the practice test and bookwork which is very helpful.
Weak and Strong Acids
Acid Disassociation
Calculating pH
Weak and Strong Acids
Acid Disassociation
Calculating pH
Mystery Substance Discovered
After the lab we completed on Tuesday, Frankenberg told us what the mystery substance was. It turns out that it was the aspirin that a few of our classmates made during the first half of the year. It was pretty cool to use the aspirin in another lab.



Lab 2
On Monday and Tuesday we did another lab titrating a mystery substance. Our average amount of KHP used was 6.00 mL. My partner and I figured out our percent error which was around 8%. Tomorrow we find out what the mystery substance is. 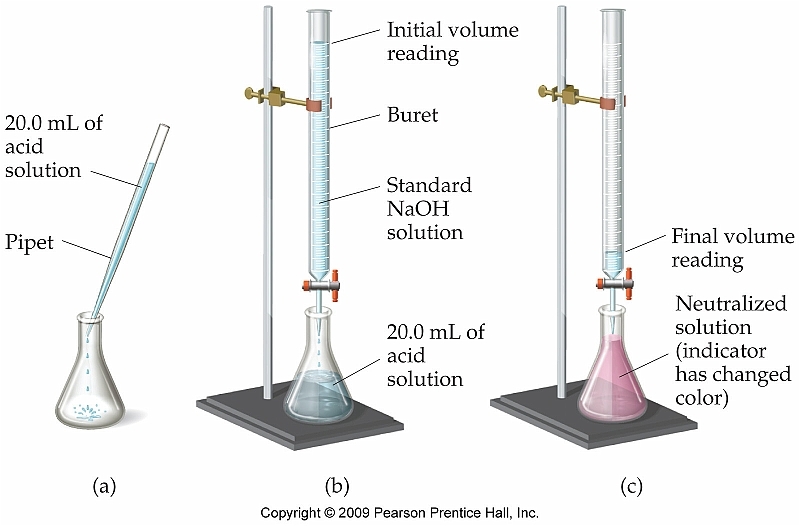

Titration
pH Calculation
Acids and Bases
Titration
pH Calculation
Acids and Bases
Vinegar Titration Lab
In class we spent three days titrating vinegar and KHP to determine the molarity of acetic acid. In order to do this we had to standardize NaOH and then titrate vinegar. It was very tedious getting the perfect shade of pink, but my partner and I managed to get a percent error of just 1.3%.
Tuesday, February 9, 2016
Study Time!
After the quiz we took I realized I need to study a little more. I did prepare for the quiz, but I was preoccupied when we took it and now I am looking to improve my score. For the exam I am going to try and read the book and work on some practice problems. I also like to complete the practice exams because they are very helpful in solidifying the concepts we learn in class.
Subscribe to:
Comments (Atom)