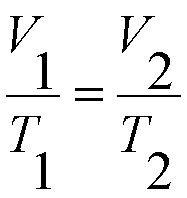Pre-AP Chem
Sunday, May 8, 2016
Quiz
We had the quiz last Thursday and I didn't think it was too difficult. I hope that the test goes well and that I don't mix up any of the gas laws. To study I will continue to do the practice test and read the book.
Avogadro's Law
The third gas law we learned about was Avogadro's Law. This law shows that gas at constant volume and pressure the volume is directly proportional to the number of moles present. The formula for this equation is V1n2=V2n1.

Avogadros Law
Extra Info

Avogadros Law
Extra Info
Charles Law
Charles Law
More Info
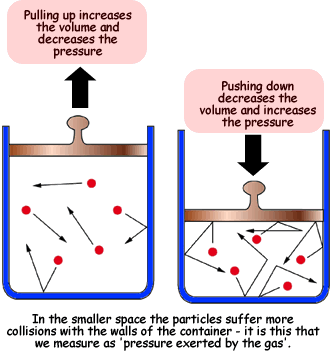
Boyle's Law
The first gas law we learned about was Boyle's Law. In Boyle's Law the temperature has to stay constant for it to be true. The equation is P1V1=P2PV2, as volume increases the pressure decreases.

Boyle's Law
More About Boyle's Law

Boyle's Law
More About Boyle's Law
Specific Heat Lab
In class we tested the specific heat of copper and lead. My partner and I tested Copper. We boiled water and placed the test tube with copper in it and let it sit for about ten minutes. We then took the test tube out and placed it in a cup of water and took the temperature of the water.

Specific Heat
Energy Of Phase Changes
Calculating Specific Heat
Specific Heat
Energy Of Phase Changes
Calculating Specific Heat
Videos
They finally released who made it to the top 10 for their biodiesel projects. Three people from our school made it to the top 10! Congratulations to everyone who made it, the videos were fun to make and I enjoyed learning about biodiesel and making it!
Subscribe to:
Comments (Atom)