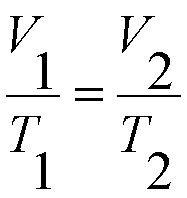Sunday, May 8, 2016
Quiz
We had the quiz last Thursday and I didn't think it was too difficult. I hope that the test goes well and that I don't mix up any of the gas laws. To study I will continue to do the practice test and read the book.
Avogadro's Law
The third gas law we learned about was Avogadro's Law. This law shows that gas at constant volume and pressure the volume is directly proportional to the number of moles present. The formula for this equation is V1n2=V2n1.

Avogadros Law
Extra Info

Avogadros Law
Extra Info
Charles Law
Charles Law
More Info
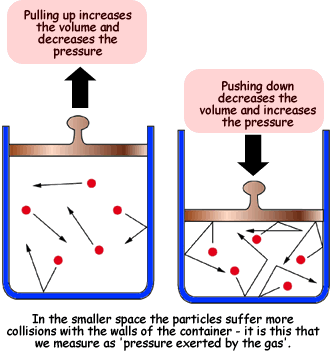
Boyle's Law
The first gas law we learned about was Boyle's Law. In Boyle's Law the temperature has to stay constant for it to be true. The equation is P1V1=P2PV2, as volume increases the pressure decreases.

Boyle's Law
More About Boyle's Law

Boyle's Law
More About Boyle's Law
Specific Heat Lab
In class we tested the specific heat of copper and lead. My partner and I tested Copper. We boiled water and placed the test tube with copper in it and let it sit for about ten minutes. We then took the test tube out and placed it in a cup of water and took the temperature of the water.

Specific Heat
Energy Of Phase Changes
Calculating Specific Heat
Specific Heat
Energy Of Phase Changes
Calculating Specific Heat
Videos
They finally released who made it to the top 10 for their biodiesel projects. Three people from our school made it to the top 10! Congratulations to everyone who made it, the videos were fun to make and I enjoyed learning about biodiesel and making it!
Boat Races and Making Biodiesel
In class we got to make biodiesel using old fryer oil from Chick-fil-a. The lab was fun and it was cool to see how simple biodiesel is to make. After we made the biodiesel, we made boats to use the biodiesel and race. Our boat worked pretty well and made it down the track in about 10 seconds.


Making Biodiesel
Alternative Fuel

Making Biodiesel
Alternative Fuel
Biodiesel
Throughout the week we have been working on videos to help promote biodiesel. The videos mostly contain info and facts about the benefits of biodiesel. Biodiesel is a cheap and energy efficient fuel alternative to replace typical fuels.


Biodiesel in Missouri
National Biodiesel
Fuel Economy
Biodiesel Basics
Biodiesel in Missouri
National Biodiesel
Fuel Economy
Biodiesel Basics
Tuesday, May 3, 2016
Hybridization
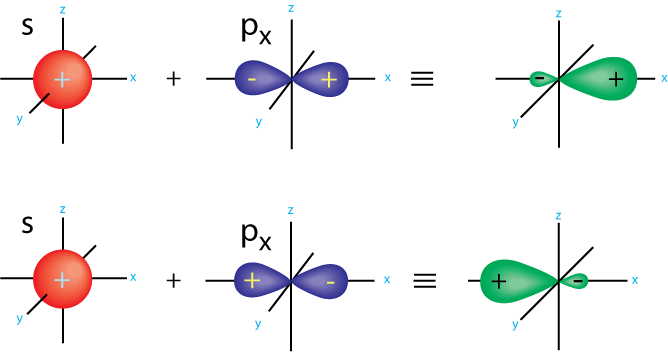
Hybridization occurs when an atoms orbitals mix to from new orbitals. The new orbitals contain the same number of electrons as the original, hybridized orbitals are an average of the unhybridized ones.
Bonding
Hybridization

Bonding
Hybridization
Molecular and Electron Geometry
Molecular geometry is the 3 dimensional arrangement of an atom while electron geometry includes valence electrons. Only bonded pairs are considered when determining an atoms molecular geometry. In electron geometry lone pairs and bonding electrons are considered.

Molecular and Electron Geometry
Molecular Geometry
Difference between Molecular and Electron Video

Molecular and Electron Geometry
Molecular Geometry
Difference between Molecular and Electron Video
Lewis Structures
 In class we learned about Lewis Structures or Electron dot diagrams. These diagrams are used to show bonds between atoms and the lone pairs of electrons. Along with Lewis Structures we learned about resonance structures. Resonance structures express the different ways a chemical bond can be drawn and represented.
In class we learned about Lewis Structures or Electron dot diagrams. These diagrams are used to show bonds between atoms and the lone pairs of electrons. Along with Lewis Structures we learned about resonance structures. Resonance structures express the different ways a chemical bond can be drawn and represented. 
Drawing Resonance Structures
Lewis Structure
Monday, March 7, 2016
Test Day!
After taking the test today I am definitely glad I read the book and completed all the practice test Frankenberg offered. This unit was easier than most we have done and it was a nice break from the difficult things we have been learning. I hope every did well on there test and that the next unit is just as interesting!
Review For The Test
Here are a few links to help review for the test! I also found the practices quizzes and worksheets very helpful on Schoology!
Electron Affinity
Quantum Numbers and Electron Configuration
Ionization Energy
Wavelength
Electron Affinity
Quantum Numbers and Electron Configuration
Ionization Energy
Wavelength
Spec 20 Lab
My partner was not here to help me with the spec 20 lab so Mrs. Frankenberg was my partner. In this lab we had to test the %T and absorbance of a Chromium solution and Cobalt solution. The lab was very time consuming, but it was cool to see the results!
Wavelength and Energy
In class we learned how to calculate wavelength and frequency. The formula is rather simple: energy of light=wavelength*frequency. The speed of light is 3.0x10^8. By knowing this formula and number it was easy to calculate the wavelength or frequency when asked. Also we learned that violet has the shortest wavelength and highest frequency of all visible light.
https://2012grade10.wikispaces.com/Spectroscopy?responseToken=50034edbd8f91eb0a5089185ef7aa9d1
https://www.youtube.com/watch?v=tIxqRSUDG6s
Wavelength and Frequency Practice
Wavelength Equation
https://2012grade10.wikispaces.com/Spectroscopy?responseToken=50034edbd8f91eb0a5089185ef7aa9d1
https://www.youtube.com/watch?v=tIxqRSUDG6s
Wavelength and Frequency Practice
Wavelength Equation
Electron Configuration
Today we got to color in class! We did this in order to show the different sections on the periodic table. These sections include s,p,d, and f. The s sublevel holds 1 orbital and can contain 2 electrons, the p sublevel holds 3 orbitals which each orbital can contain 6 electrons, the d subshell holds 5 orbitals and can contain 10 electrons, and lastly the f subshell holds 7 orbitals and can contain 14 electrons. These different subshells help to determine an elements electron configuration which helps to locate it on the periodic table.
Flame Test Lab
We did a flame test lab in class to see what color different metals burned at. It was sometimes difficult to see the exact color of the flame the metal produced, but it was still a really cool lab. After we finished collecting data, we had to determine the wavelength these metals burned at and the moles/photon for each metal.
Tuesday, February 16, 2016
After Test
After the test I'm glad I did the practice test. It was also very helpful having the ACT program on my calculator to help with the ICE box questions. I wish there was more time to go over questions, so I am going to work on my time management for our next test.
Extra Links for Studying
Our test is coming up and Frankenberg said it will be one of the hardest test we take. Due to this statement I have posted some extra links to help. I have also done the practice test and bookwork which is very helpful.
Weak and Strong Acids
Acid Disassociation
Calculating pH
Weak and Strong Acids
Acid Disassociation
Calculating pH
Mystery Substance Discovered
After the lab we completed on Tuesday, Frankenberg told us what the mystery substance was. It turns out that it was the aspirin that a few of our classmates made during the first half of the year. It was pretty cool to use the aspirin in another lab.



Lab 2
On Monday and Tuesday we did another lab titrating a mystery substance. Our average amount of KHP used was 6.00 mL. My partner and I figured out our percent error which was around 8%. Tomorrow we find out what the mystery substance is. 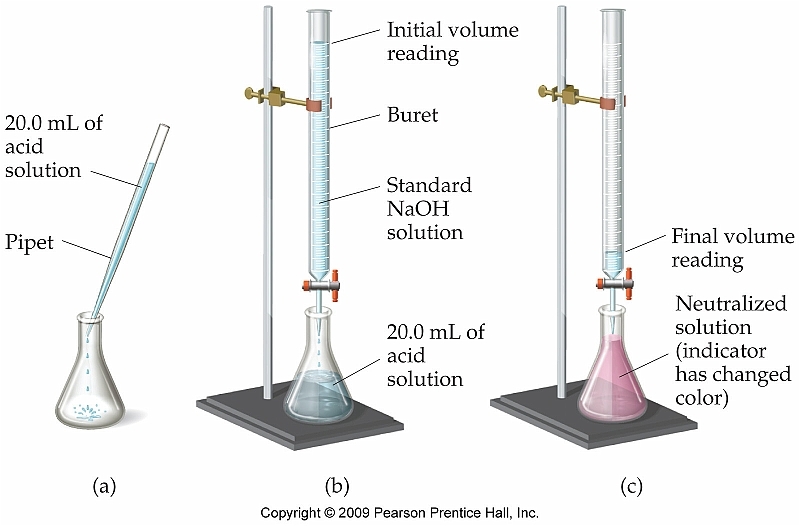

Titration
pH Calculation
Acids and Bases
Titration
pH Calculation
Acids and Bases
Vinegar Titration Lab
In class we spent three days titrating vinegar and KHP to determine the molarity of acetic acid. In order to do this we had to standardize NaOH and then titrate vinegar. It was very tedious getting the perfect shade of pink, but my partner and I managed to get a percent error of just 1.3%.
Tuesday, February 9, 2016
Study Time!
After the quiz we took I realized I need to study a little more. I did prepare for the quiz, but I was preoccupied when we took it and now I am looking to improve my score. For the exam I am going to try and read the book and work on some practice problems. I also like to complete the practice exams because they are very helpful in solidifying the concepts we learn in class.
Sunday, January 24, 2016
Murder Investigation Lab
The murder investigation lab was a great lab to practice finding the molarity of a substance. My lab partners and I mixed Silver nitrate with sodium carbonate to form a substance. We then filter out the substance known as silver carbonate and took the mass of it after it dried for a day. We then determined the molarity of the substance and determined the killer was Mr. Green.
Subscribe to:
Comments (Atom)